So I felt like I couldn’t put off the sixteen hundred articles twiddling their thumbs and tapping their feet in my RSS reader any longer. This is the first part of the crop that has accumulated since late December (yikes!). Legless axolotls, homing starfish, secretly related proteins, and more!
1. Axolotls are good at regenerating – until you make them grow up.

(Portrait of a pale lab/aquarium variety axolotl by Orizatriz, Wiki Commons.)
It’s probably not exactly obvious from my posting record, but a large part of my PhD work is about regeneration. It’s something we humans are pretty shit at, but many other vertebrates aren’t. Axolotls, these adorably dumb-faced salamanders, can easily regrow their legs. However, lab axolotls are kind of permanent babies. Although they can grow up in the sense that they are able to breed, they normally keep larval characteristics like gills throughout their lives. It’s reasonable to suspect that this influences their regenerative ability – after all, tadpoles lose their ability to regrow limbs the moment they turn into frogs.
It’s possible to make axolotls metamorphose, too, if you treat them with thyroxine (the same hormone that induces metamorphosis in “normal” amphibians). And when they turn into proper adult salamanders, they suddenly become much poorer regenerators. They can still replace a limb – kind of. But they take twice as long as non-metamorphosed axolotls of the same age and size, and they invariably wind up with small, malformed limbs, often missing bones. After amputation, new skin is slower to grow over their wounds, and the cells that gather under the new skin are sluggish to divide. Something about metamorphosis – that isn’t simply age – dramatically changes how they respond to amputation.
Reference: Monaghan JR et al. (2014) Experimentally induced metamorphosis in axolotls reduces regeneration rate and fidelity. Regeneration advance online publication, doi: 10.1002/reg2.8
*
2. Similar cells repair muscles in crustaceans and vertebrates
“Regeneration” can cover a lot of different processes. For example, depending on the creature and the organ you’ve damaged, regenerated body parts can come from totally different kinds of cells. In planarian flatworms, a single kind of stem cell can replace anything else in the body. In the eyes of newts, mature cells of the iris transform into lens cells to replace a missing lens. In our muscles, there are special cells called satellite cells that are held in reserve specifically to make new muscle cells when needed.
This recent study of a little crustacean called Parhyale hawaiensis suggests that muscle regeneration in the fingernail-sized arthropod works in much the same way. Konstantinidis and Averof shot early embryos of Parhyale with DNA encoding a fluorescent marker, which randomly integrated into the genomes of some of the cells it hit. In a few “lucky” individuals, the marker ended up labelling just one cell lineage, and the pair used these animals to figure out which cells made which tissues in a regenerated limb.
It turned out that cells in Parhyale are limited in their potential. Descendants of the ectodermal lineage could make skin and nerves but not muscle, and the mesodermal lineage built muscle but not skin or nerves. Moreover, labelled cells only contributed to regeneration near their original location – animals with their left sides labelled never regrew glowing limbs on the right side. This is starting to sound a lot like vertebrates, but it’s still a very general observation. However, the similarities don’t end there.
Like vertebrate muscles, the muscles of the little crustaceans contain satellite-like cells derived from the mesodermal lineage that sit beside mature muscle cells and express the Pax3/7 gene. When the researchers transplanted some of these cells from animals with the glowy label into leg stumps of non-glowy animals, there were glowing muscle cells in some of the regenerated limbs. So like satellite cells, these cells can turn into muscle during regeneration. There’s little question that muscle cells have a common origin in vertebrates and arthropods like Parhyale, but it’s really cool to see that their mechanisms of regeneration also might.
Reference: Konstantinidis N & Averof M (2014) A common cellular basis for muscle regeneration in arthropods and vertebrates. Science, published online 02/01/2014, doi: 10.1126/science.1243529
*
3. Convergent evolution is a poor explanation of rhodopsins
Proteins can be difficult. I mean, sometimes they do their darnedest to hide their family ties. A protein is a chain of amino acids (on average about 300 of them) often folded into a complex shape. Closely related proteins have obviously similar amino acid sequences. However, more distant relatives can be harder to identify. There are about 20 different kinds of amino acids in proteins, so the number of possible sequences is unimaginably vast. The same function can be carried out by very different sequences, and therefore enough evolution can completely erase sequence similarity.
Protein structures are generally thought to be more conserved than sequences. Like function, structure allows for a huge amount of sequence variation without significantly changing. However, theoretically, it’s possible that two unrelated proteins have similar structures because of their similar functions, not because of common ancestry. Apparently, this has been argued for the two types of rhodopsins – proteins that harvest light in systems as different as a the “solar generator” of a salt-loving microbe and the photoreceptors of our own eyes.
If Type I and Type II rhodopsins are similar despite being unrelated, one would assume that this is because they need to be that way to capture light. There are, after all, astronomical numbers of possible protein structures, and the chances of two protein families accidentally stumbling onto the same one without selection steering are slim to say the least. But, in fact, you can rearrange the structure of a rhodopsin in all kinds of cunning ways without destroying its function. This rather weakens the case for convergent evolution, and suggests that similarity of structure does indicate common ancestry here.
Reference: Mackin KA et al. (2014) An empirical test of convergent evolution in rhodopsins. Molecular Biology and Evolution 31:85-95
*
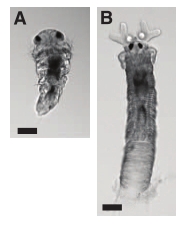
4. Starfish can see their way back home
(Blue starfish, the beast featured in the paper, in its natural habitat. Richard Ling, Wiki Commons.)
Starfish aren’t widely known as visual creatures, but they do have eyes at the tips of their arms. The eyes are a bit… basic – no lenses, just a hundred or two little units filled with photoreceptors. Garm and Nilsson set out to find out how the starfish used their eyes. They measured or calculated the eyes’ visual fields (five arm-eyes together can see pretty much everywhere around the animal), resolution (very coarse), reaction speed (slow), and their sensitivity to various wavelengths (they are colour-blind, most sensitive to ocean blue).
Then they took some poor starfish and dumped them a little way off the coral reefs they like staying on. The creatures could walk home from short distances (about 2 m or less), but if you take them too far away, they just wander around in random directions. Likewise if you take off their eyes (don’t worry, they regenerate) or do the experiment in the dark. In conclusion: starfish eyes aren’t exactly top-end cameras, but they are definitely useful to the animals. And what would a slow, brainless mopper-up of coral reef rubbish do with eagle eyes anyway?
(The paper states the walking speed of these starfish as about 4-5 cm per minute. I have a feeling this wasn’t the most exciting fieldwork these guys have done…)
Reference: Garm A & Nilsson D-E (2014) Visual navigation in starfish: first evidence for the use of vision and eyes in starfish. Proceedings of the Royal Society B 281:20133011
*
5. What makes wormies settle
OK, Shikuma et al. (2014) one isn’t so much for its own news value, but I hadn’t known that my favourite worms need bacteria to settle until I saw this paper, so I think it deserves a mention. (Besides, it has beautiful pictures of baby Hydroides in it, which I couldn’t resist posting below. They are So. Cute. Yes, I’m weird.)
Tubeworms of the serpulid family have swimming larvae which are in many ways like the acorn worm larvae mentioned in my previous post (except cuter). They are tiny, look nothing like an adult worm, have bands of cilia for swimming and feeding, and live in the plankton until they’re ready to metamorphose. When they find a place they like, they settle and turn into adult worms. And apparently, this particular species (Hydroides elegans) not only needs a specific bacterium to like a place, it needs specific proteins produced by that bacterium.
The proteins in question are the components of a nasty device bacteria probably stole from viruses and then used to poke holes in one another. But to Hydroides larvae, they appear to be necessary for metamorphosis. Put healthy bacteria together with worm babies in a dish, and you’ll get happily settled little worms. Do the same with bacteria with damage to the relevant genes, and nothing happens. Use an extract containing the proteins but not the bacteria, and you still get metamorphosing worms. Use too much, though, and they start dying. Everything in moderation…
(Maybe my dismal failure at raising happy young worms years ago could have been remedied with the right bacteria?)
Reference: Shikuma NJ et al. (2014) Marine tubeworm metamorphosis induced by arrays of bacterial phage tail-like structures. Science 343:529-533
*
6. Relative of animals does strange multicellularity with familiar genetics
Although this idea probably hasn’t reached popular perception, animals are surrounded by other multicellular lineages in the tree of life. Sure, most of them are only part-time multicellular, but that’s beside the point. What’s clear is that multicellularity, at least in its simpler forms, is rampant in our extended family. Slime moulds do it, fungi do it, our closest relatives choanoflagellates do it, and our next closest relatives, filastereans and ichthyosporeans also do it.
These latter two groups are really poorly known (the fact that only a taxonomist could like the latter’s name probably doesn’t help), but the situation is getting better with the attention they are receiving as relatives of animals. There are now genome sequences out, and some people are looking at the life cycles of the little creatures to search for clues to our own origins.
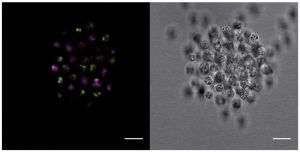
Iñaki Ruiz-Trillo recently published a paper describing an ichthyosporean that can form a weird kind of colony with many nuclei in the same membrane starting from a single cell (Suga and Ruiz-Trillo, 2013). Now his team describe a different kind of multicellularity in a filasterean, Capsaspora owczarzaki. Rather than developing from a single cell, this guy does something more akin to the slime mould way: take a load of individual cells and bring them together. (Below: a clump of Capsaspora cells from Sebé-Pedros et al. [2013]. On the right is a regular photograph of the colony. The two-coloured fluorescence on the left indicates that the colony formed by different cells coming together rather than a single cell dividing.)
But, interestingly, some of the genetics involved is similar to what animals use, despite the different ways in which the two groups achieve multicellularity. For example, we’ve known since all those genomes came out that the proteins animals use to glue cells together and make them talk to each other are often older than animals. Well, Ruiz-Trillo’s filasterean appears to ramp up the production of some of these when it goes multicellular. It also uses a gene regulation strategy that animals are really big on: it edits the RNA transcribed from many genes in different ways depending on cell type/life stage before it’s translated into protein.
A lot of the details are going to need further investigation, since this was a global RNA-sequencing study with a bird’s-eye view of what genes are doing. It’s still a nice reminder that, like most other innovations in evolutionary history, the multicellularity of animals didn’t spring fully formed out of nowhere.
References:
Suga H & Ruiz-Trillo I (2013) Development of ichthyosporeans sheds light on the origin of metazoan multicellularity. Development 377:284-292
Sebé-Pedros A et al. (2013) Regulated aggregative multicellularity in a close unicellular relative of metazoa. eLife 2:e01287