I didn’t plan to write anything today, but damn, Cambrian explosion. And polychaetes. I can’t not. Plus I’m going on holiday soon, so I might as well get something in before I potentially disappear off the internet. (Below: a Cambrian polychaete, Canadia spinosa, via the Smithsonian’s Burgess Shale pages.)

First, a confession.
I’m a bit of a coward about the Cambrian explosion.
Make no mistake, I love it. It’s fascinated me ever since I came across the heavily Stephen Jay Gould-flavoured account in The Book of Life. It’s an event that made the world into what it is today, with its complex ecosystems full of animals eating, cooperating or competing with each other. And it’s one of the great mysteries of palaeontology. What actually happened? What caused it? Why did it happen when it did? Why didn’t it happen again when animal life was nearly wiped out at the end of the Permian?
The problem is, I love it so much that I’m afraid to have an opinion about it. You have no idea how many times I wanted to discuss the big questions, only to shy away for fear of getting it wrong. Which is really kinda stupid, because no one has the one and only correct answer. Whether I’m qualified to comment on it is a different issue, but it wouldn’t be the first subject I comment on that I don’t fully understand.
So, here I take a deep breath and plunge into Sperling et al. (2013).
The abstract started by scaring me. It begins, “The Proterozoic-Cambrian transition records the appearance of essentially all animal body plans (phyla), yet to date no single hypothesis adequately explains both the timing of the event…” To which my immediate reaction was “why the fuck would you want a single hypothesis to explain it?” But luckily, they don’t. They actually argue for a combination of two hypotheses, which they think are more connected than we thought.
But let’s just briefly establish what the Cambrian explosion is.
I want to make this absolutely clear: it’s not the sudden appearance of modern animals out ot nowhere. It could be more accurately described as the appearance of basic body plans we traditionally classify as phyla, such as echinoderms, molluscs, or arthropods, in a relatively short geological period.
Doug Erwin (2011) trawled databases and literature to draw up a timeline of first appearances for animal phyla, and he found that they increase in number gradually over a period of 80 million years (see Erwin’s plot below).
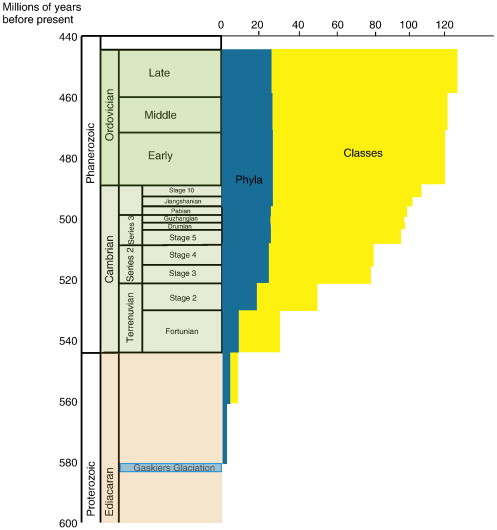
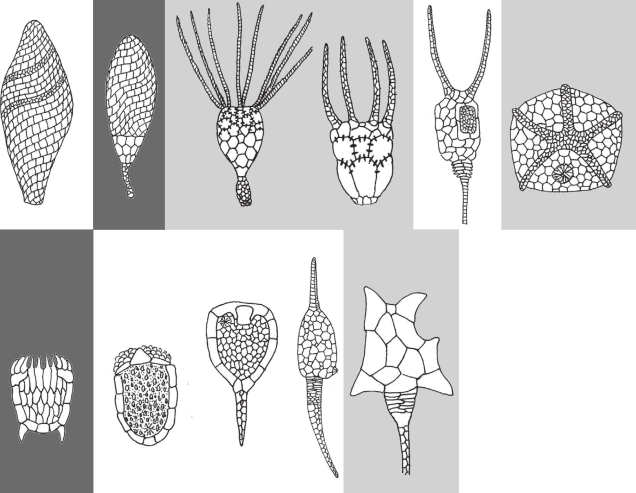
Appearance of “phyla” also doesn’t equal appearance of modern animals, as Graham Budd has been known to emphasise (e.g. Budd and Jensen, 2000). For example, I already mentioned how none of the mid-Cambrian echinoderms recently described by Smith et al. (2013) would look familiar today. In fact, the modern classes of echinoderms, which include sea urchins, starfish and sea lilies, didn’t appear until after the Cambrian. Likewise, while there were chordates (our own phylum), and probably even vertebrates, in the Cambrian, such important vertebrate features as jaws or paired appendages were yet to be invented. (If memory serves, both of those inventions date to the Silurian.)
There is also a discussion to be had about the meaning and validity of concepts like a phylum or a body plan, but let’s not complicate things here. I have a paper to get to! 🙂
With that out of the way…
There is no doubt that something significant happened shortly before and during the Cambrian. Before the very latest Precambrian, fossils show little evidence of movement, of predation, or of the diverse hard parts that animals use to protect themselves or eat others today. All of these become commonplace during the Cambrian, establishing essentially modern ecosystems (Dunne et al., 2008).
There are many explanations proposed to account for the revolution. I’ve not the space (or the courage) to discuss them in any detail. If you’re interested, IIRC Marshall (2006) is a very nice and balanced review. (Link leads to a free copy.) However, we can discuss what Sperling et al. have to say about two of them.
The first hypothesis is oxygen, which likely became more abundant in the ocean towards the end of the Precambrian. That could explain the timing, but maybe not the nature of the explosion. Oxygen levels impose a limit on the maximum size of animals, but what compels larger animals to “invent” more disparate body plans? (Also, on a side note, many Ediacaran organisms weren’t exactly tiny, so I’m not sure how much of a size limit there is in the first place.)
The second one is animal-on-animal predation (Sperling et al. prefer the term “carnivory”), which can lead to predator-prey arms races and therefore encourage the evolution of innovations like shells or burrowing or jaws that give one party an edge. This is a decent enough basis for body plan innovation, but it applies for any time and place with animals. So if carnivory is the explanation, why did the explosion happen when it did?
Because, Sperling et al. argue, carnivory and oxygen are linked.
I’m intrigued by their approach. They’re not looking at fossils in this study at all. (I always like it when palaeontology and the biology of the living join forces!) They are looking at oxygen-poor habitats in modern oceans. Specifically, they asked how low oxygen levels affect polychaete worm communities.
Why polychaetes? The authors give a list of reasons. One, polychaetes are really, really abundant on the seafloor, and particularly so in low-oxygen settings. Two, different species feed in almost every conceivable way from filtering plankton through chewing through sediment to flat out devouring other animals, and their feeding mode can usually be guessed even if you haven’t seen that particular species eat. Three, they are actually quite good at handling oxygen limitation. This is important because back in the Precambrian, all animals would have been well adapted to a low-oxygen environment, so a group that can tolerate the same may be the best comparison. (They do note that a previous study of a single low-oxygen site that took other animals into account came up with similar results to theirs.)
They worked partly with pre-existing datasets that met a set of criteria designed to get a complete and unbiased view of local polychaete diversity. In total, they analysed data from 68 sites together featuring nearly a thousand species of worms. They also had some of their own data.
They categorised their study sites into four levels of oxygen deprivation, and counted numbers of carnivorous individuals and species at each site. They came to the conclusion that lack of oxygen basically makes carnivores disappear. The lowest-oxygen samples contained fewer carnivores on both the individual and species levels, and they were more likely to be devoid of predators altogether (# species plot from the paper below):
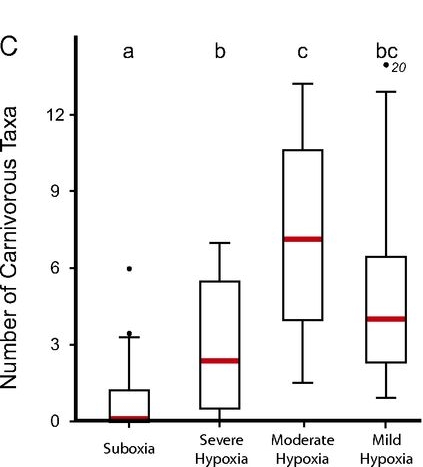
There are a couple of different ways in which lack of oxygen could limit predators. For example, the aforementioned size limit is one, because it’s good for a predator to be larger and stronger than its prey. But the biggest factor according to the authors is the energy required for an active predatory lifestyle. While a suspension feeder can sit in one place all day and only move to stuff a food-laden tentacle into its mouth, a predator has to find, subdue and eat its prey, which are all pretty expensive activities. Then it also has to digest a sudden, large meal, whereas the suspension feeder’s digestion works at a low and steady rate. Animals can get energy from a variety of metabolic processes, but by far the most efficient route requires oxygen. And that really sucks when you are a hunter who might need large amounts of energy at short notice.
Hmm…
Although I’m quite intrigued by the study, there are a couple of issues that bother me. For example, as far as I could tell, all of the study sites included in the analyses were low on oxygen. I would have liked to see them compared to “normal” sites, in particular because the trend in predator abundance wasn’t a neat straight upwards line. In fact, the least oxygen-deprived habitats appeared less predator-infested than slightly more oxygen-poor ones. What’s going on there?
In terms of interpretation in relation to the Cambrian, I also would have liked to see a comparison of the oxygen levels at their study sites to what’s estimated for the geological periods in question. I take it they just didn’t have precise enough estimates, because one of the things they discuss in the closing paragraph is the need to measure just how much oxygen went into the oceans during this late Precambrian oxygen increase.
And my semi-silly question is, how does this apply to “predators” who don’t run around chasing after and wrestling with prey? For example, sea anemones might be perfectly happy to eat large creatures. But they don’t really do much. They just sit and wait, and if a poor fish stumbles onto their sticky venomous tentacles, tough luck for it. Or there’s the unknown predator that drilled holes in late Precambrian Cloudina specimens (Bengtson and Zhao, 1992). Cloudina was sessile, the creature didn’t have to chase it… Predators such as these still have to cope with the energy demands of digesting sudden large meals, I suppose, so maybe the energetics idea still applies. And of course, if there’s no oxygen, large prey is less likely to be swimming around bumping into your tentacles.
Is this “the” explanation of the Cambrian explosion? Probably not, says the cynic in me. I highly doubt we’re done with that question. Is it a good explanation? Well, it is certainly evidence-based, and I like it that it tries to take different factors together and in context. What I don’t think it does is explain the uniqueness of the Cambrian. A thousand words or so ago, I mentioned the Permian extinction. That cataclysm very nearly left the earth devoid of animals. Afterwards, there was certainly enough oxygen for predators to thrive in the sea, and indeed they did, from sea urchins to ichthyosaurs. So why didn’t the first 40 million years of the Mesozoic era beget many new phyla the way the first 40 million years of the Palaeozoic did? Is that just an artefact of our classifications or was something really fundamentally different going on?
I ain’t Jon Snow, but when it comes to the Cambrian, I still feel like I know nothing…
***
References:
Bengtson S & Zhao Y (1992) Predatorial borings in Late Precambrian mineralized exoskeletons. Science 257:367-369
Budd GE & Jensen S (2000) A critical reappraisal of the fossil record of the bilaterian phyla. Biological Reviews 75:253-295
Dunne JA et al. (2008) Compilation and network analyses of cambrian food webs. PLoS Biology 6:e102
Erwin DH (2011) Evolutionary uniformitarianism. Developmental Biology 357:27-34
Marshall CR (2006) Explaining the Cambrian “explosion” of animals. Annual Review of Earth and Planetary Sciences 34:355-384
Smith AB et al. (2013) The oldest echinoderm faunas from Gondwana show that echinoderm body plan diversification was rapid. Nature Communications 4:1385
Sperling EA et al. (2013) Oxygen, ecology, and the Cambrian radiation of animals. PNAS 110:13446-13451